Agrobacterium-Mediated Gene Transfer
Agrobacterium tumefaciens is a soil bacterium causing crown gall tumours on several dicotyledonous plant species, e.g. tobacco or Arabidopsis.
Tumours are formed at sites where wounding has occurred. The bacterium induces tumour growth by genetic transformation of the plant cell (Fig. 222).
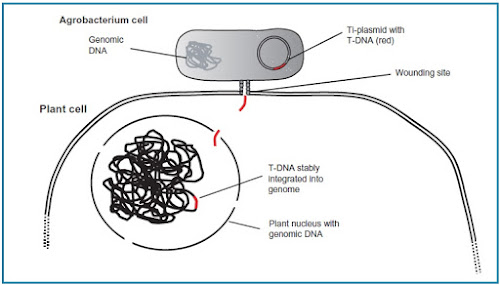 |
| Fig. 222 : T-DNA transfer from Agrobacterium to the plant nucleus and integration into the genomic plant DNA |
At wounding sites plant cells release small compounds such as phenolic acetosyringone. This is perceived by the bacterium, which then activates genes localized on the so-called tumour-inducing (Ti) plasmid (circular DNA molecule).
The activation results in the release of a DNA fragment from the Ti plasmid, called Transfer-DNA or T-DNA. The T-DNA stably integrates into the plant genome.
It contains genetic information to modify the hormone levels in the plant, causing tumour development.
For genetic engineering of plants the Ti plasmid had to be disarmed so that it no longer induced tumours.
The tumour-causing genes of Agrobacterium wild-type strains that are located on the T-DNA are now replaced by the gene of interest and a selectable marker gene.
As with biolistic gene transfer, Agrobacterium-mediated gene transfer is carried out using immature wheat embryos.
Agrobacterium-mediated transformation of cereals using immature embryos as explants first succeeded in 1994 with rice (Hiei et al., 1994); it was followed by maize two years later (Ishida et al., 1996) and finally by barley and wheat in 1997 (Tingay et al., 1997; Cheng et al., 1997).
A major advantage of using Agrobacterium for transformation is the higher rate of single copy insertions compared to the biolistic method.
The selectable marker genes co-transformed with the gene of interest can be the same as the ones used in the biolistic transformation method, mediating antibiotic or herbicide resistance of transformed cells.
A new generation of selectable marker genes enables transformed cells to metabolize nutrients that plants cannot normally utilize.
For example, marker genes encoding the enzymes xylose isomerase or mannose phosphate isomerase allow selection with sugars like xylose or mannose respectively. Non-transformed cells starve to death, as no carbon source is available to them.
Both DNA transfer methods are used for genetic transformation of wheat, but a review of the scientific literature up to 2003 reveals that most foreign genes are transferred into wheat by microprojectile bombardment (Sahrawat et al., 2003).
The reasons for this are two-fold.
First the transformation of wheat by the bombardment method preceded Agrobacteriummediated transformation by five years, so the technique was well established in many laboratories.
Secondly, it was difficult to establish the Agrobacterium-mediated transformation system for wheat in different laboratories where different wheat genotypes are used.
Different Agrobacterium strains show different degrees of effectiveness on different wheat varieties. Conditions for the co-cultivation of the bacterium with the wheat tissue require optimization, whereas the purely physical method using the particle gun does not call for the adjustment of two organisms.
The limitation for generating transgenic wheat plants is not so much the gene transfer technique as the in vitro culture performance of many wheat varieties.
The rate of callus induction and subsequent regeneration is an intrinsic factor of a variety, and so far it cannot usually be overcome by modifying the culture conditions.
The problem is circumvented by screening for wheat varieties with a high regeneration efficiency in order to study the function of a gene of interest.
Wheat and other cereals are more than just model systems for functional studies, unlike practical model plants like Arabidopsis.
With the increasing use of agronomically important transgenes, commercial wheat varieties will become the focus of genetic improvement by means of gene transfer, and it can be predicted that efforts will increase to improve their regeneration efficiency.
Bol Registration
ReplyDeleteBol Game Show Registration
Bol Game Show Aisay Chalega Registration
bol qurandazi winner 2020
bol show aisay chalega registration 2020
bol qurandazi winner list
bol card winners list 2020
bol qurandazi winner
game show aisay chalega registration 2020
bachatwala card game show 2020
Bol Game Show
Post a Comment